Research
(+) |Energy Storage and Conversion| (-)
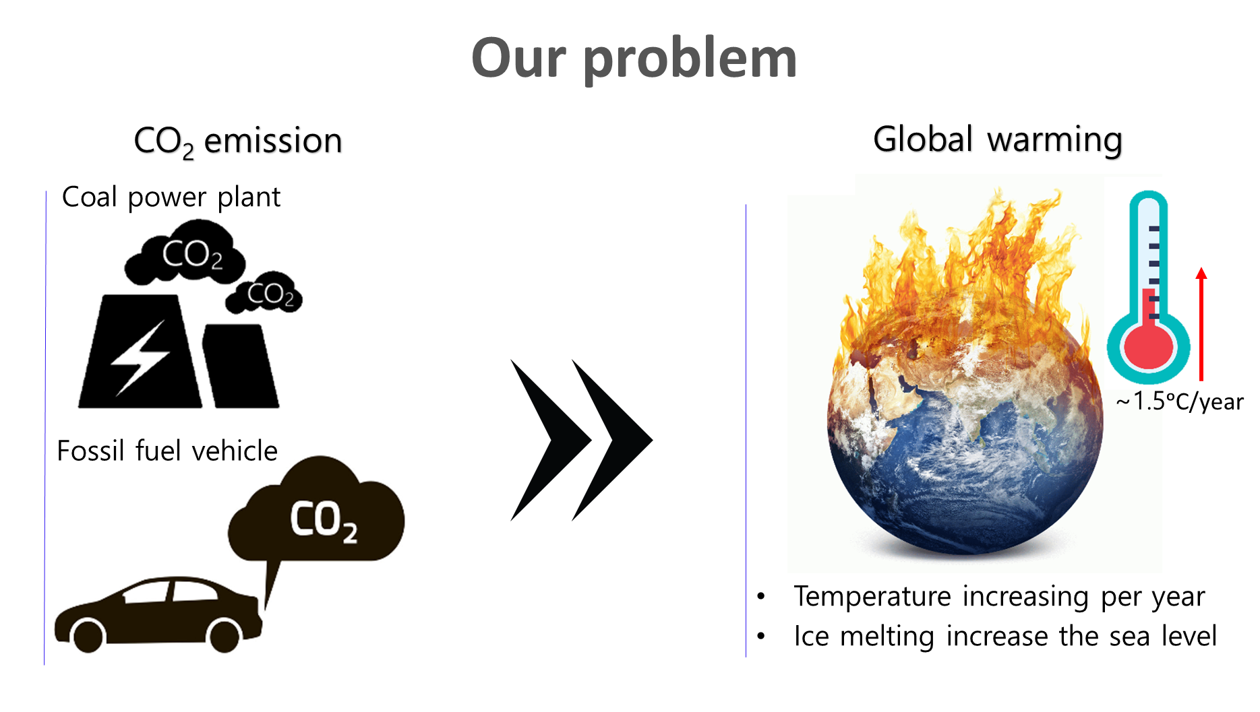
Carbon dioxide (CO2) is the primary greenhouse gas responsible for heating the atmosphere and oceans, leading to more extreme weather events and rising sea levels. Approximately 80% of global CO2 emissions stem from burning coal, oil, and gas, with coal used in power stations and oil and gas being significant sources for vehicles. As a result, many countries are now reducing fossil fuel power plants by transitioning to renewable energy sources such as wind and solar power. Additionally, they are replacing oil and gas-based vehicles with electric vehicles (EVs). However, we require cost-effective, user-friendly, low-maintenance energy storage systems with a long lifespan to facilitate the widespread adoption of intermittent renewable energy sources and EVs.
My research activities focus on energy storage materials and developing energy storage and conversion systems, including seawater batteries, redox flow batteries, and supercapacitors, to meet the abovementioned requirements.
1. Batteries:
(i). Seawater battery:
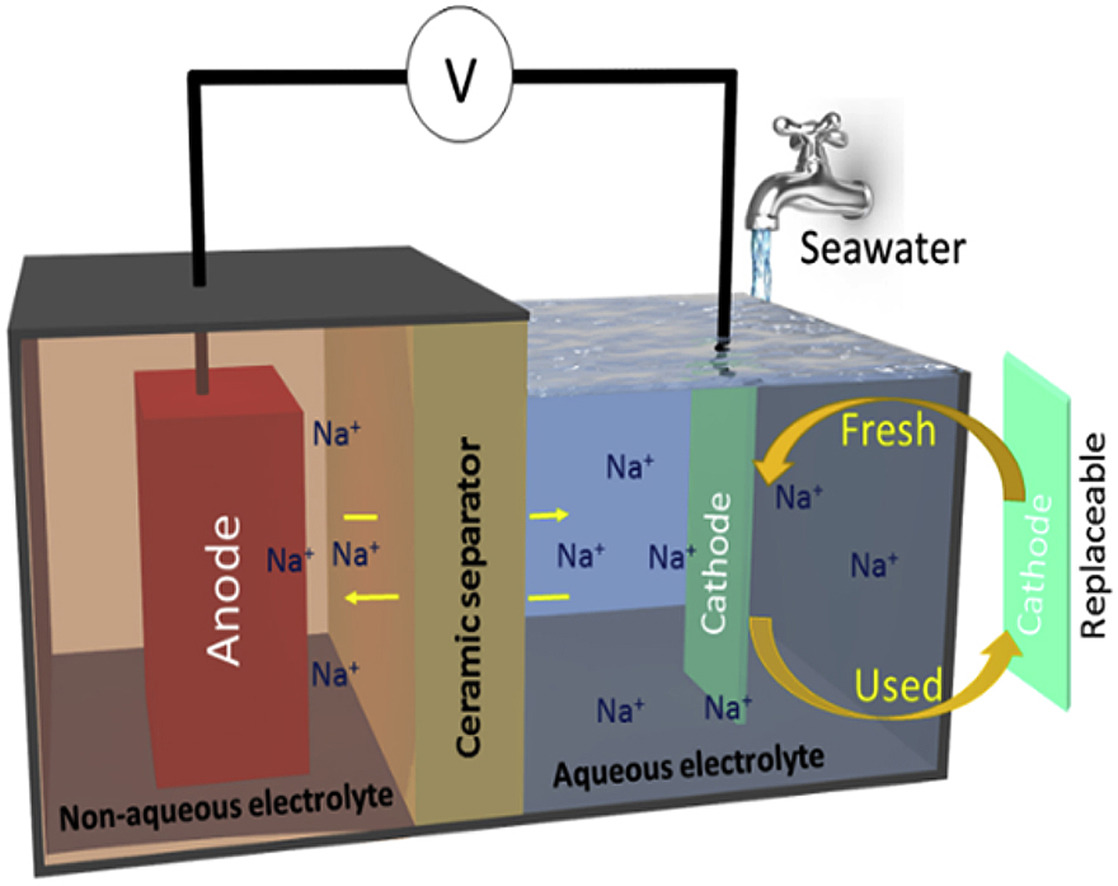
Seawater batteries (SWBs) could be a promising contender for large-scale implementation as their eco-friendly and use of abundant seawater as sources of Na-ions or active materials. This SWB can convert chemical energy into electrical energy, often represented as reversible like rechargeable metal (Li/Na)-air batteries. But unlike rechargeable metal (Li/Na)-air/O2 batteries, SWB typically utilizes the Na-ions, hydroxide ions, H2O molecules, and dissolved oxygen present in the seawater for the repeated charge-discharge process.
Related Publications:
1. Development of coin-type cell and engineering of its compartments for rechargeable seawater batteries, J Han, SM Hwang, W Go, S. T. Senthilkumar, D Jeon, Y Kim. Journal of Power Sources, 2018, 374, 24-30. (Link)
2. Seawater battery performance enhancement enabled by a defect/edge- rich, oxygen self-doped porous carbon electrocatalyst, S. T. Senthilkumar, Sung O. Park, Junsoo Kim, Soo Min Hwang, Sang Kyu Kwak and Youngsik Kim. Journal of Materials Chemistry A, 2017, 5, 14174-14181. (Link)
3. Emergency of Rechargeable Seawater Battery, S. T. Senthilkumar; W. Go; J. Han; L. P.Thi Thuy; K. Kishor; Y.Kim; Y. Kim. Journal of Materials Chemistry A, 2019,7, 22803-22825 (Review article). (Link)


(ii). Hybrid electrolyte Na-ion battery:
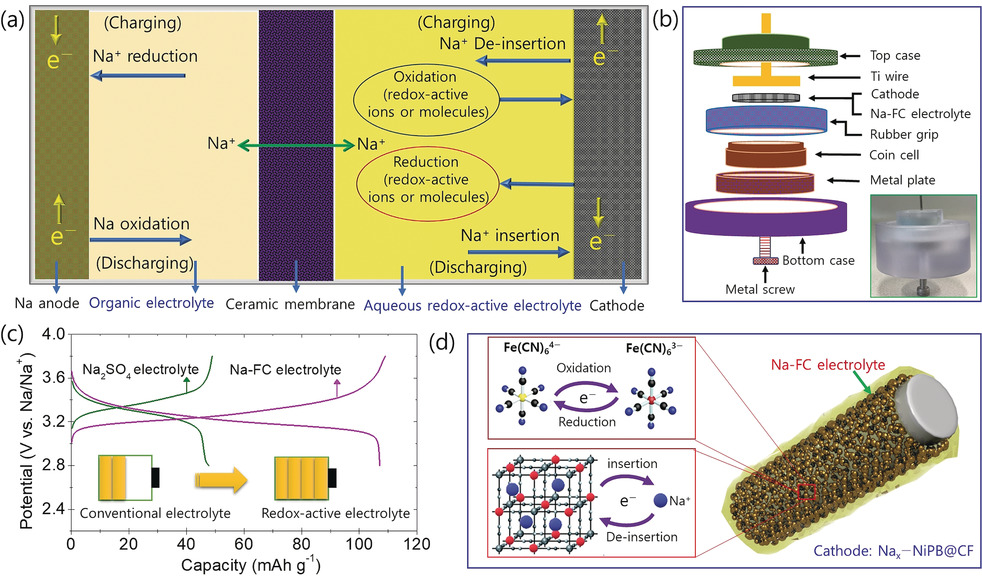
Aqueous rechargeable sodium-ion batteries (ARSBs) have attracted much attention as a promising alternative owing to advantages such as low cost, green, and safety. However, one of the primary disadvantages of ARSBs is that they deliver a relatively low energy density owing to the limited working voltage (∼2 V) due to the decomposition of water. So, the enhancement of cell voltage has been a major challenge. However, the working voltage of ARSBs can be improved by using NASICON (Na3Zr2Si2PO12) as the solid electrolyte, which separates the moisture-sensitive Na metal anode (in a non-aqueous electrolyte) from the aqueous electrolyte (water).
Related Publications:
1. Sodium-ion hybrid electrolyte battery for sustainable energy storage applications, S. T. Senthilkumar; M. Abirami; J. Kim; W. Go; S. M. Hwang. Y. Kim. Journal of Power Sources, 2017, 341, 404-410. (Link)
2. Enhancing capacity performance by utilizing the redox chemistry of the electrolyte in a dual-electrolyte sodium-ion battery, S. T. Senthilkumar; H. Bae; J. Han; Y. Kim. Angewandte Chemie, 2018, 130, 5433-5437. (Link)
(iii). Hybrid electrolyte Na-ion Flow battery:
Redox flow battery (RFB) technologies have become a significant role in the future in storing electrical energy produced from intermittent renewable energies such as solar, wind, and hydroelectric powers. However, the development of high-energy-density RFB remains challenging. The energy density can be increased in two ways; (i) using high concentration and increasing cell voltage. Na-aqueous-catholyte RFB (NaAqRFB) could be a potential contender for high-density electrical energy storage. NASICON (Na3Zr2Si2PO12) is employed as a solid electrolyte in the NaAqRFB to separate the Na anode and a flowable aqueous catholyte. In NaAqRFB, the Na-metal anode can offer a high capacity and lower redox potential, which is key to increasing the cell voltage and energy density. The use of an aqueous flowing catholyte in NaAqRFB could decouple the energy and power.
Related Publications:
1. Energy efficient Na-aqueous-catholyte redox flow battery, S. T. Senthilkumar; J. Han; J. Park; S. M. Hwang; D. Jeon; Y. Kim. Energy Storage Materials, 2018, 12, 324-330. (Link)
2. Supercapacitors:
(i). Fiber-type weaveable supercapacitor:
Flexible fiber electronic devices have attracted extensive research interest in the past few years due to their unique features and potential applications in next-generation wearable electronics and smart textiles. Various fiber-type energy storage devices are highly demanded to store or power the available fiber electronic devices. Among them, supercapacitors (SCs) act as a promising candidate that can offer long cycle life, high power density, safety, etc.
Related Publications:
1· Advances and prospects of fiber supercapacitors, S. T. Senthilkumar; Y. Wang; H. Huang. Journal of Materials Chemistry A, 2015, 3, 20863-20879 (Review article). (Link)
2· Flexible and wearable fiber shaped high voltage supercapacitor based on copper hexacyanoferrate and porous carbon coated carbon fiber electrode, S. T. Senthilkumar; J. Kim; Y. Wang; H. Huang; Y. Kim. Journal of Materials Chemistry A, 2016, 4, 4934-4949. (Link)
3· Flexible fiber hybrid supercapacitor with NiCo2O4 nanograss@carbon fiber and bio-waste derived high surface area porous, S. T. Senthilkumar; N. Fu; Y. Liu; Y. Wang; L. Zhou; H. Huang. Electrochimica Acta, 2016, 211, 411-419. (Link)
(ii). Redox electrolyte supercapacitors:
Supercapacitors (SCs) are a promising field in energy storage devices. In the last few decades, different types of carbon-based materials with suitable surface modifications, metal oxides, metal hydroxides, conducting polymers, and their various composites, have been used as electrodes to improve the energy performance of SCs. In addition, different technologies like asymmetric and hybrid systems have also been introduced. Interestingly, another alternative approach has been proposed recently by a few research groups, wherein electrolytes (liquid and polymer) can enhance the performance of the SCs via redox reactions at the electrode-electrolyte interface by introducing redox additives or mediators in the electrolytes. The main advantage of this new technique is its safe and straightforward preparation method and cost-effectiveness compared to the preparation of some active electrode materials. Hence, it is believed that identifying suitable redox additives or species in electrolytes will be a hotspot in the field of SCs in the coming years.


Related Publications:
1· Redox additive/active electrolytes: A novel approach to enhance the performance of Supercapacitors, S. T. Senthilkumar; R. Kalai Selvan; J. S. Melo. Journal of Materials Chemistry A, 2013, 1, 12386-12394 (Feature Article). (Link)
2· High performance solid-state electric double layer capacitor from redox mediated gel polymer electrolyte and renewable tamarind fruit shell derived porous carbon, S. T. Senthilkumar; R. Kalai Selvan; J. S. Melo; C. Sanjeeviraja. ACS Applied Materials & Interfaces, 2013, 5, 10541-10550. (Link)
3· Improved performance of electric double layer capacitor using redox additive (VO2+/VO2+) aqueous electrolyte, S. T. Senthilkumar; R. Kalai Selvan; N. Ponpandian; J. S. Melo; Y. S. Lee. Journal of Materials Chemistry A, 2013, 1, 7913-7919. (Link)